Welcome to Kardio-Med Silesia Site
Silesian Park of Medical Technology Kardio-Med Silesia
is a modern research center meet the highest European standards.
 The direct contact method according to PN-EN ISO 10993, Part 5 is one of the test methods for in vitro cytotoxicity by direct contact between the test material and the cell culture. This procedure is commonly used to assess the biocompatibility of biomaterials/medical devices, and is a qualitative method for determining cytotoxicity effects.
The direct contact method according to PN-EN ISO 10993, Part 5 is one of the test methods for in vitro cytotoxicity by direct contact between the test material and the cell culture. This procedure is commonly used to assess the biocompatibility of biomaterials/medical devices, and is a qualitative method for determining cytotoxicity effects.
In vitro testing can be considered as the first step to determine biocompatibility of biomaterial/ medical device. An undoubted advantage is the low cost of conducting this type of testing, and the results obtained enable analysis of the initial cellular response to the test material in a short period of time. Meanwhile, the absence of a negative cellular response in the cytotoxicity test makes it possible to start the in vivo testing phase, including testing of acute, subacute, subchronic and chronic toxicity, irritation and sensitization, or implantation in tissues.
The in vitro cytotoxicity testing procedure, in accordance with the PN-EN ISO 10993-5 group standard “In vitro cytotoxicity testing“, is carried out within the infrastructure of the Cell Laboratory, which is part of the infrastructure of the Multispecialty Laboratory of the Silesian Park of Medical Technologies Kardio-Med Silesia. The test method involves aplying the test material (a piece of biomaterial or medical device) directly on a cell layer, derived from the L-929 cell line, and exposing them to the test material for at least 24h, at 37 ± 1°C and under conditions of 5 ± 1% CO2 atmosphere. Figure 1 illustratively presents a single well of the culture plate, during in vitro cytotoxicity testing by direct contact method.
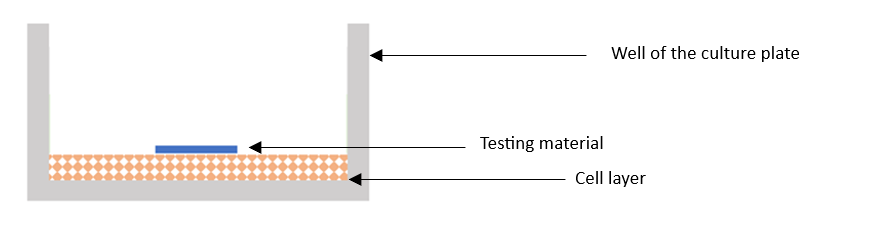
Fig. no 1. Visualization of the well of the culture plate in the direct contact cytotoxicity test, according to PN-EN ISO 10993-5 “Biological evaluation of medical devices – Part 5: In vitro cytotoxicity tests“.
 Evaluation of the cytotoxic effect of the test material on the cells is performed using a fluorescence microscope, which makes it possible to visualize the state of the cells immediately before and after the removal of the test material, based on the descriptive guidelines in PN-EN ISO 10993-5. The biological reactivity of cells is evaluated on a scale from grade 0 (no reactivity) to grade 4 (high reactivity); a medical device is assumed to be non-cytotoxic if none of the cultures exposed to the test material shows a level higher than grade 2 (mild reactivity).
Evaluation of the cytotoxic effect of the test material on the cells is performed using a fluorescence microscope, which makes it possible to visualize the state of the cells immediately before and after the removal of the test material, based on the descriptive guidelines in PN-EN ISO 10993-5. The biological reactivity of cells is evaluated on a scale from grade 0 (no reactivity) to grade 4 (high reactivity); a medical device is assumed to be non-cytotoxic if none of the cultures exposed to the test material shows a level higher than grade 2 (mild reactivity).
The selection of the appropriate method for testing the cytotoxicity of biomaterials or medical devices depends on the type(s) of biomaterials used, the shape or the target application of the medical device. Please feel free to contact our specialists to select the appropriate test method according to your needs.
The laboratories of the Silesian Park of Medical Technologies Kardio-Med Silesia are covered by the Good Laboratory Practice compliance certificate for biocompatibility tests (i.e., scope No. 9, as defined by the Decree of the Minister of Health of August 3, 2021, on Good Laboratory Practice and performance of tests in accordance with the principles of Good Laboratory Practice), including in vitro cytotoxicity tests.







Copyright 2023 Kardio-Med Silesia. Site designed by Daniel 'zoNE' Gabryś. All rights reserved.