Welcome to Kardio-Med Silesia Site
Silesian Park of Medical Technology Kardio-Med Silesia
is a modern research center meet the highest European standards.
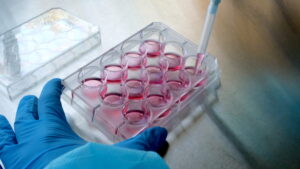 In vitro testing refers to research conducted outside of a living organism, usually in laboratories, on isolated cells, tissues or organs. In vitro testing allows the study of various biological processes, chemical interactions, toxicity of substances and other aspects, without the use of living organisms.
In vitro testing refers to research conducted outside of a living organism, usually in laboratories, on isolated cells, tissues or organs. In vitro testing allows the study of various biological processes, chemical interactions, toxicity of substances and other aspects, without the use of living organisms.
Cytotoxicity testing, allows testing the ability of chemicals to damage or destroy cells. The elution method in this case means elucidating what mechanisms underlie this cell damage.
At Our Testing Unit, testing is carried out in a cellular laboratory, both with and without Good Laboratory Practice standards. International standards and guidelines for conducting laboratory tests in accordance with GLP and ISO 10993 ensure a high level of quality, accuracy and reliability of results. These guidelines are designed to ensure the integrity and quality of laboratory data that are generated for scientific and regulatory purposes.
Elution methods for cytotoxicity testing may include:
In vitro, elution-based cytotoxicity tests are important because they provide reliability and transparency of results, which is crucial in assessing the safety of chemicals, medical devices, pharmaceuticals and other products that may come into contact with the human body.







Copyright 2023 Kardio-Med Silesia. Site designed by Daniel 'zoNE' Gabryś. All rights reserved.