Welcome to Kardio-Med Silesia Site
Silesian Park of Medical Technology Kardio-Med Silesia
is a modern research center meet the highest European standards.
 We encourage you to get acquainted with Our offer in the field of biological evaluation of medical devices in accordance with the requirements of ISO PN-EN 10993, in the Good Laboratory Practice (GLP) standard, and with the offer of a wide range of in vitro and in vivo tests, individually tailored to your needs and the specifics and requirements of the test protocol!
We encourage you to get acquainted with Our offer in the field of biological evaluation of medical devices in accordance with the requirements of ISO PN-EN 10993, in the Good Laboratory Practice (GLP) standard, and with the offer of a wide range of in vitro and in vivo tests, individually tailored to your needs and the specifics and requirements of the test protocol!
Research is carried out in Our Unit using modern and specialized equipment, allowing us to carry out research both within in vitro systems (on cellular models) and in vivo – in small and large animal models, with special care and the highest quality, ensured, among other things, by the certificate of compliance with the principles of Good Laboratory Practice (GLP) issued by the Bureau of Chemical Substances, obtained by the Silesian Park of Medical Technology Kardio-Med Silesia in August 2022. The foundation of modern biotechnology and medicine is to ensure the safety of a medical device, medicinal product or other chemical substances that can adversely affect human health and life. Therefore, regulatory agencies such as the Office for the Registration of Medicinal Products, Medical Devices and Biocidal Products (URPL) and the European Medicines Agency (EMA) place great emphasis on carrying out manufacturing and production work based on guidelines and regulations.
Conducting research using cell lines, such as assessing biocompatibility or toxicity at the in vitro level, makes it possible to check under laboratory conditions whether the use of a given substance, medical device or medicinal product is safe and what effect a given test material has on the transformations taking place inside the cells of the human body. Conducting cellular research makes it possible to reduce the number of experimental animals used for experiments, enables more conclusive results to be obtained and, at the same time, is financially economical, which ultimately has a positive impact on the company’s budget. However, despite continuous work by international cooperation bodies such as the FDA and the OECD, it has not been possible to develop research methods that will completely eliminate the use of experimental animals for research.
In vivo research, that is, research in a small- or large-animal model, can range from biological evaluation studies of medical devices conforming to standards in the ISO PN-EN 10993 group, to a variety of other research protocols, including an immunocompromised mouse model, which is used for oncology drug trials, or a domestic pig model dedicated to cardiac procedures, as well as urology or ophthalmology. In addition, thanks to its state-of-the-art infrastructure, highly specialized apparatus and qualified and experienced staff of specialists, including veterinarians, zootechnicians, biotechnologists and specialists in interventional medicine. The Silesian Park of Medical Technology Kardio-Med Silesia has the ability to conduct research on a research model using mice, rats, rabbits, guinea pig and domestic pigs and mini pigs.
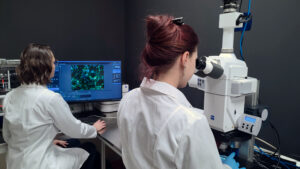 Multispecialty laboratories, including the Cell Laboratory dedicated to in vitro research, or the living quarters of small and large animal models for in vivo research, are equipped with apparatus for culture and observation of cell systems, preparation of reference and test materials, histological analysis, or monitoring of environmental conditions (such as temperature, humidity). Included in the Multispecialty Laboratory, is the Microscopic Imaging Laboratory, where accurate evaluation of cells is carried out, as well as high-end equipment that gives the opportunity to carry out professional research and benefit from the extensive capabilities of optical equipment. As part of the infrastructure of the large-animal animal facility, mention should be made, among other things, of a high-end operating theater with modern equipment for performing advanced research procedures in an in vivo model, including apparatus for extracorporeal circulation, full equipment for inhalation anesthesia, and an angiograph, for intraoperative or postoperative observations. In the small animal room, there is equipment to evaluate the effectiveness of drug therapy under development, such as inhalation. With such an advanced infrastructure, Our Center is able to offer a wide range of tests both under the quality system of Good Laboratory Practice confirmed by certification, as well as under non-certified tests.
Multispecialty laboratories, including the Cell Laboratory dedicated to in vitro research, or the living quarters of small and large animal models for in vivo research, are equipped with apparatus for culture and observation of cell systems, preparation of reference and test materials, histological analysis, or monitoring of environmental conditions (such as temperature, humidity). Included in the Multispecialty Laboratory, is the Microscopic Imaging Laboratory, where accurate evaluation of cells is carried out, as well as high-end equipment that gives the opportunity to carry out professional research and benefit from the extensive capabilities of optical equipment. As part of the infrastructure of the large-animal animal facility, mention should be made, among other things, of a high-end operating theater with modern equipment for performing advanced research procedures in an in vivo model, including apparatus for extracorporeal circulation, full equipment for inhalation anesthesia, and an angiograph, for intraoperative or postoperative observations. In the small animal room, there is equipment to evaluate the effectiveness of drug therapy under development, such as inhalation. With such an advanced infrastructure, Our Center is able to offer a wide range of tests both under the quality system of Good Laboratory Practice confirmed by certification, as well as under non-certified tests.
We carry out ongoing in vitro studies on cell models and in vivo studies on small and large animal models in a very wide range, and we have high availability of free appointments and competitive prices.
Contact us and we will prepare a tailor-made offer for you!







Copyright 2023 Kardio-Med Silesia. Site designed by Daniel 'zoNE' Gabryś. All rights reserved.