Welcome to Kardio-Med Silesia Site
Silesian Park of Medical Technology Kardio-Med Silesia
is a modern research center meet the highest European standards.
The agar diffusion method according to ISO 10993 part 5 is one of the methods of in vitro cytotoxicity testing by indirect contact of the test sample with the agar. The procedure is used for the biological evaluation of medical devices using the L-929 cell line.
 Proceeding in accordance with the procedure developed on the basis of ISO 10993 “Biological evaluation of medical devices – Part 5: In vitro cytotoxicity tests” allows for a qualitative assessment of the cytotoxicity of the tested sample, i.e. a medical device. His assay is not appropriate for leachables that cannot diffuse through the agar layer, or that may react with agar.
Proceeding in accordance with the procedure developed on the basis of ISO 10993 “Biological evaluation of medical devices – Part 5: In vitro cytotoxicity tests” allows for a qualitative assessment of the cytotoxicity of the tested sample, i.e. a medical device. His assay is not appropriate for leachables that cannot diffuse through the agar layer, or that may react with agar.
The procedure for testing the cytotoxic effect in vitro using the agar diffusion method according to ISO 10993 part 5 is carried out in a cell laboratory and consists in placing agar with cell medium on a layer of L-929 cells of the appropriate confluence. The test sample is applied to the top layer of agar. The whole is incubated for 24 – 26 hours at 37 ± 1°C in 5 ± 1% CO2. Figure 1 shows the appearance of the well of the culture plate, where at the very bottom there are cells, then the agar on which the test sample is placed.
The cytotoxic effect is read after a day of incubation of the tested sample with cells and agar. In this case, the condition of the cells should be assessed under a microscope immediately before and after the removal of the test sample, based on the descriptive guidelines contained in the ISO 10993 – 5 standard.
The biological assessment of a medical device in terms of its cytotoxic effect on cells seems to be the observation of the state of cells under a microscope. The cytotoxic effect of the test sample on the cell line is determined on the basis of a scale, where 0 means no effect of the test sample on cells, and 4 means a serious cytotoxic reaction in vitro. The achievement of a number grade greater than 2 (mild reactive), based on the guidelines of the ISO 10993 – 5 standard, is considered a cytotoxic effect of the tested medical device.
Cytotoxicity testing by an indirect contact method such as agar diffusion is validated test procedure. However, it may not be accepted by all supervisory committees.
We invite you to contact us directly to select the appropriate research method for your medical device.
Our laboratory has a certificate of Good Laboratory Practice in the part of biocompatibility (range 9) for the biological assessment of medical devices for research, among others in vitro cytotoxicity.
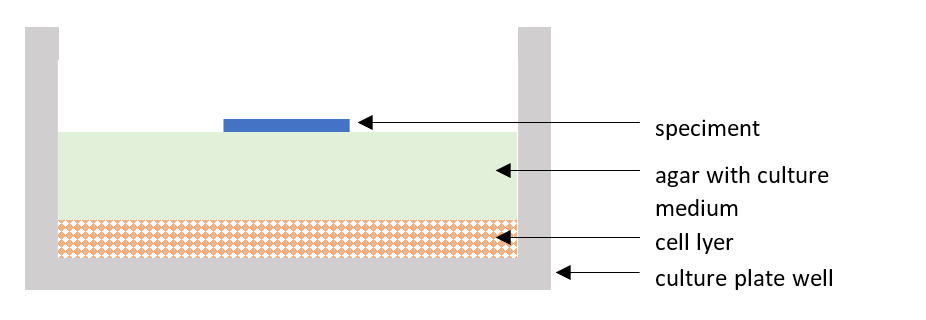
Figure 1 Visualization of the culture plate well in cytotoxicity test – agar diffusion assay according to ISO 10993-5 “Biological evaluation of medical devices – part 5: Tests for in vitro cytotoxicity”.







Copyright 2023 Kardio-Med Silesia. Site designed by Daniel 'zoNE' Gabryś. All rights reserved.