Welcome to Kardio-Med Silesia Site
Silesian Park of Medical Technology Kardio-Med Silesia
is a modern research center meet the highest European standards.
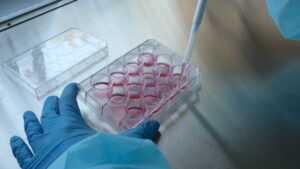 In vitro testing is an extremely important part of the process of evaluating the safety and efficacy of medical devices before they are placed on the market. The term “in vitro” refers to laboratory experiments conducted outside a living organism, as opposed to “in vivo” studies, which take place in living organisms such as laboratory animals or humans.
In vitro testing is an extremely important part of the process of evaluating the safety and efficacy of medical devices before they are placed on the market. The term “in vitro” refers to laboratory experiments conducted outside a living organism, as opposed to “in vivo” studies, which take place in living organisms such as laboratory animals or humans.
In vitro testing is crucial in evaluating the potential adverse effects and toxicity of medical devices, as well as their efficacy. One of the main aspects governing in vitro testing is the principle of Good Laboratory Practices (GLP), which is an international standard that ensures the reliability, consistency and precision of results. With GLP, in vitro tests are conducted according to well-defined procedures and documented in detail, enabling repeatability of results.
 During in vitro testing, cells or tissues are exposed to chemicals, medical devices or therapeutics and then monitored to assess whether they produce the desired therapeutic effects or potential adverse effects. This approach allows researchers to identify potential risks and improve the efficacy of products before they reach the market. In vitro testing also allows for the study of the mechanisms of action of medical devices at the molecular level, which is key to understanding their effects on the body.
During in vitro testing, cells or tissues are exposed to chemicals, medical devices or therapeutics and then monitored to assess whether they produce the desired therapeutic effects or potential adverse effects. This approach allows researchers to identify potential risks and improve the efficacy of products before they reach the market. In vitro testing also allows for the study of the mechanisms of action of medical devices at the molecular level, which is key to understanding their effects on the body.
In conclusion, in vitro testing is an indispensable step in the process of evaluating medical devices, allowing for the meticulous study of their properties and effects on the body, in accordance with GLP principles, which contributes to ensuring the safety and efficacy of medical products on the market.
The Silesian Park of Medical Technologies Kardio-Med Silesia is certified by Good Laboratory Practice in the biocompatibility (scope 9) for biological evaluation of medical devices for in vitro cytotoxicity testing, among others.
Feel free to contact us to match the appropriate testing method to your medical device.







Copyright 2023 Kardio-Med Silesia. Site designed by Daniel 'zoNE' Gabryś. All rights reserved.