Welcome to Kardio-Med Silesia Site
Silesian Park of Medical Technology Kardio-Med Silesia
is a modern research center meet the highest European standards.
In vivo studies play a key role in evaluating the safety, efficacy and performance of medical devices before they are put on the market. The term “in vivo” refers to experiments conducted in living organisms, such as laboratory animals or humans, as opposed to “in vitro” studies, which take place outside the body.
One of the main tenets of conducting in vivo research in our company is to follow the principles of Good Laboratory Practices (GLP), which guarantee the accuracy, reproducibility and reliability of results. Thanks to GLP, in vivo studies are planned, conducted and documented in a well-defined manner, which enables reliable assessment of the effects of medical devices on living organisms.
Different animal models are used in in vivo studies, depending on the purpose of the study and the specifics of the product. Small animal models, such as mice or rats, are often used for initial toxicity and efficacy studies. Large animal models, such as the domestic pig, are more similar to the human body in terms of anatomy and physiology, allowing for more reliable predictions of medical device performance in humans.
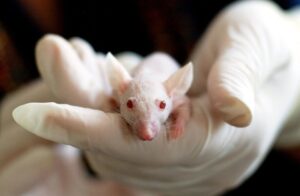 In vivo studies allow assessment of the effects of medical products on the entire body, including organ systems and biological processes. They can be used to identify potential side effects, assess the bioavailability of active substances and monitor long-term therapeutic effects.
In vivo studies allow assessment of the effects of medical products on the entire body, including organ systems and biological processes. They can be used to identify potential side effects, assess the bioavailability of active substances and monitor long-term therapeutic effects.
 In conclusion, in vivo testing is an indispensable step in the evaluation process of medical devices, providing reliable and comprehensive information on their effects on living organisms. By using small- and large-animal models and adhering to GLP principles, it is possible to better understand the effects of medical devices and ensure their safety and efficacy before access to patients.
In conclusion, in vivo testing is an indispensable step in the evaluation process of medical devices, providing reliable and comprehensive information on their effects on living organisms. By using small- and large-animal models and adhering to GLP principles, it is possible to better understand the effects of medical devices and ensure their safety and efficacy before access to patients.
The Silesian Park of Medical Technologies Kardio-Med Silesia is certified by Good Laboratory Practice in the biocompatibility (scope 9) for biological evaluation of medical devices for in vitro testing, among others.
Feel free to contact us to match the appropriate testing method to your medical device.







Copyright 2023 Kardio-Med Silesia. Site designed by Daniel 'zoNE' Gabryś. All rights reserved.