Welcome to Kardio-Med Silesia Site
Silesian Park of Medical Technology Kardio-Med Silesia
is a modern research center meet the highest European standards.
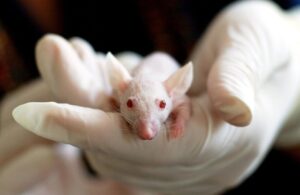 Acute toxicity testing is an extremely important step in the process of evaluating the safety and biocompatibility of medical devices and drugs. It is a key step in ensuring that medical products and drugs that reach the market are safe for both patients and medical personnel.
Acute toxicity testing is an extremely important step in the process of evaluating the safety and biocompatibility of medical devices and drugs. It is a key step in ensuring that medical products and drugs that reach the market are safe for both patients and medical personnel.
Acute toxicity testing is one of the fundamental components of biological evaluation of medical devices and drug safety testing. It aims to identify potential toxic hazards that may occur after the body comes into contact with a given medical product or drug. The key objective of this test is to evaluate the effects of a medical device or drug on the body over a short period of time, immediately after exposure.
 Acute toxicity testing procedures are strictly defined by ISO 10993 standards, OECD guidelines and MDR regulations, which are part of the current legislation. These tests must be conducted in accordance with Good Laboratory Practice (GLP) standards, and the results have a significant impact on decisions on the approval and registration of medical devices or drugs.
Acute toxicity testing procedures are strictly defined by ISO 10993 standards, OECD guidelines and MDR regulations, which are part of the current legislation. These tests must be conducted in accordance with Good Laboratory Practice (GLP) standards, and the results have a significant impact on decisions on the approval and registration of medical devices or drugs.
The stages of toxicity testing are acute, subacute, subchronic and chronic toxicity. Each of these stages is designed to evaluate the effects of the body’s exposure to the test product over different periods of time.
The histology laboratory plays a key role in acute toxicity studies. Specialists analyze tissue samples taken in studies to assess morphological and structural changes in tissues. These observations are extremely important in determining the effects of a product on the body.
Conclusions from acute toxicity studies are of great importance in the process of evaluating the safety and biocompatibility of medical devices and drugs. Based on them, decisions are made regarding further stages of testing, product approval and marketing. Accurate analysis and interpretation of toxicity test data, in accordance with current regulations and standards, is extremely important when registering medical devices and marketing new drugs. It is worth emphasizing that acute toxicity testing is an integral part of the process of ensuring the safety and efficacy of medical devices and drugs prior to their use in medical practice.







Copyright 2023 Kardio-Med Silesia. Site designed by Daniel 'zoNE' Gabryś. All rights reserved.